Prof. Sharmistha Sinha
Professor, Scientist-F, Dean Academics, Former Head CBU
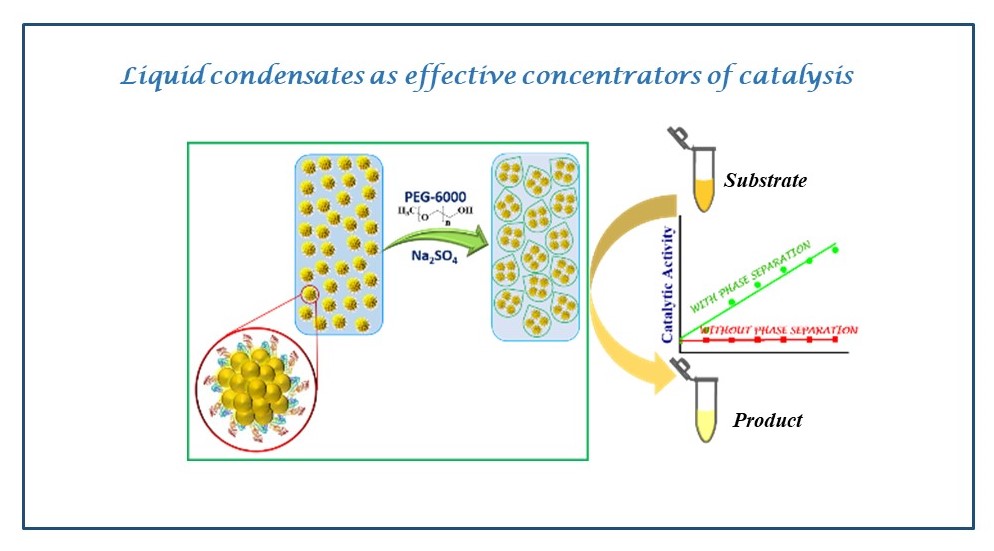
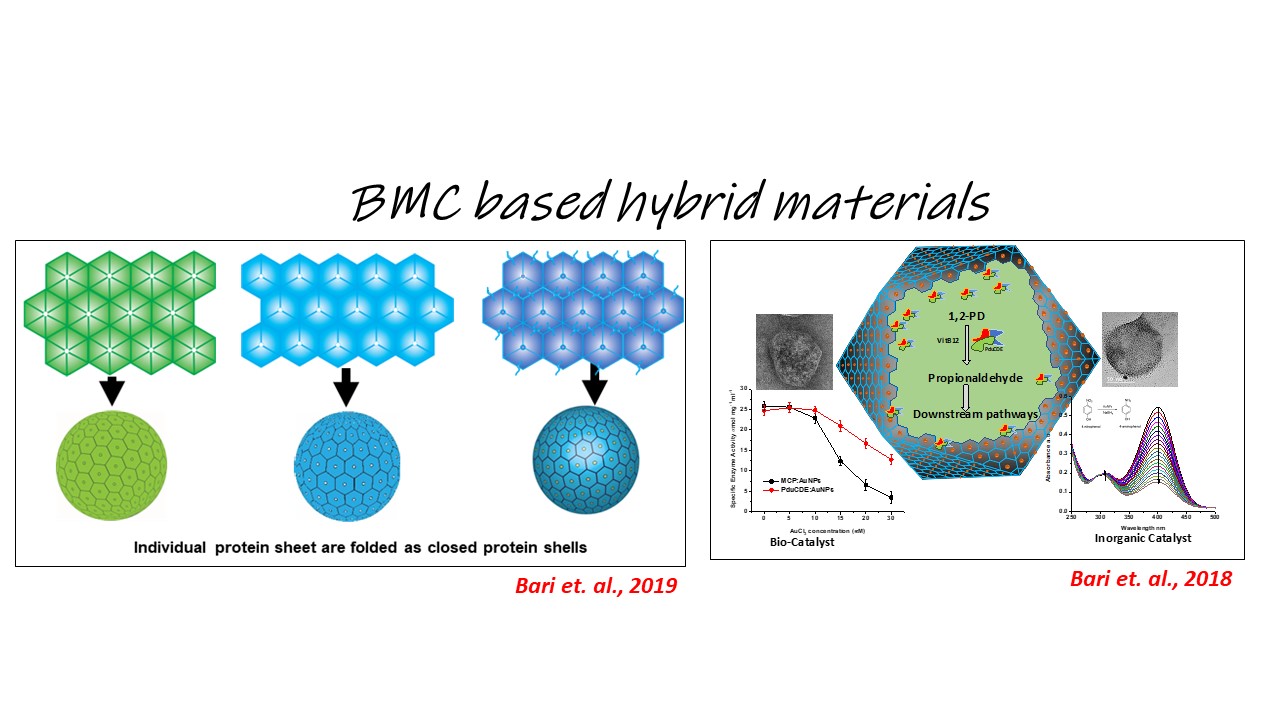
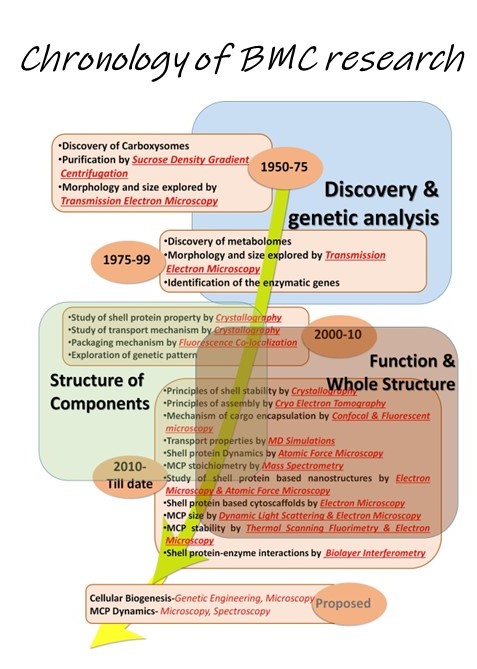
Bacterial microcompartments (BMCs) are unique paradigms of prokaryotic organelles that encapsulate certain metabolic reactions under specific microbial physiology. The Sinha lab is actively engaged in understanding the biogenesis pathogenesis in the bacterial microcompartments associalted with Salmonella enterica. The lab is partcularly interexted in the following concepts: BMCs as models to explore compartmentalization in biology and organelle biogenesis in Salmonella BMCs as targets to manage Salmonella BMCs as scaffolds for the development of smart nano-factories and nanomaterials The Sinha Lab is looking for highly motivated students eager to work in the interdisciplinary areas of disease biology and chemistry. Interested students may write to sinhas@inst.ac.in. Students with independent scholar recognition via CSIR/DBT/DST fellowships are welcome to apply.
Contact Information :
-
E-mail:
sinhas@inst.ac.in -
Google Link:
Google Link